What Does the Principal Quantum Number Determine
What does the magnetic quantum number determine. What does the principal quantum number determine.
Were always here.

. Chemistry questions and answers. What does the principal quantum number determine. A principal quantum number n indicates the principal electron shell which will indicate the distance of the electrons from the nucleus.
What does Hunds rule state. The orientation of the orbital. The magnetic quantum number can be derived from solving the azimuthal equation of the hydrogen Schrodinger equation.
The energy of the state. What does the Magnetic Quantum Number Determine. Four different quantum numbers are used in quantum mechanics.
Shells and Subshells of Orbitals. What does the principal quantum number determine. For the hydrogenlike atom the principal quantum number n determines.
The larger value of n corresponds to the average distance of an electron in. That when more than one orbital of equal energy is available electrons will first occupy these orbitals singly with parallel spins. What letter is the magnetic quantum number.
In fact the energy is proportional to -1n2 so two states with the same n have the same energy and vice versa. The magnetic quantum number primarily determines the number of orbitals and the orientation of orbitals in a given sub-shell. The size and energy of the orbital.
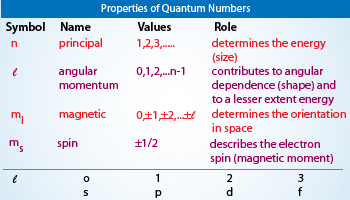
What letter is the principal quantum number. As the value of n increases the energy of the orbital increases. Consequently it is dependent on the orbital angular.
The principal quantum number gives a look into the electron density around a nucleus. What does the principal quantum number determine. Principal quantum number n determines the size of an orbital.
The l value specifies the shape of the orbital. For example in caesium Cs the outermost valence electron is in the shell with energy level 6 so an electron in caesium can have an n. Principle quantum number n Principal Quantum Number n specifies the energy of an electron and the size of the orbital in an atom.
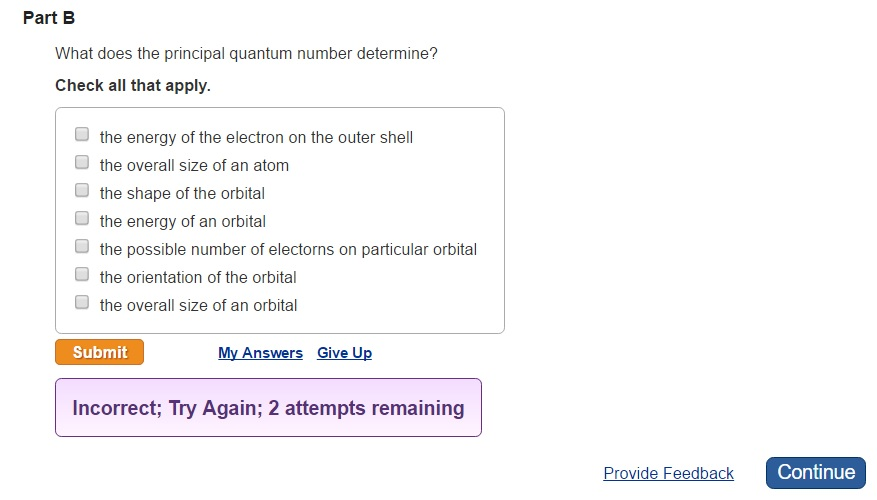
Its values are natural numbers from 1 making it a discrete variable. The energy of an orbital the possible number of electorns on particular orbital the overall size of an atom the overall size of an orbital the shape of the orbital the energy of the electron on the outer shell. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
Overall size and energy of an orbital. This doesnt hold in multi-electron atoms though. It has integral values ie.
Check all that apply. N 1 2 3 n. N 1 2 3 4.
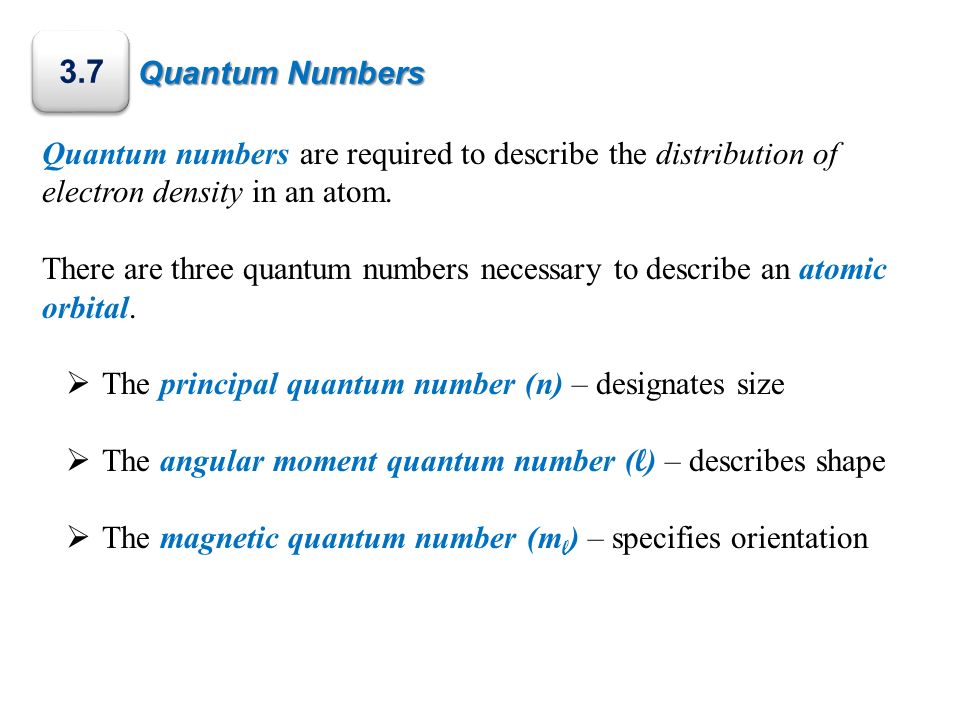
The Principal Quantum Number. The first of those quantum numbers is cal. The principle quantum number n is an integer that determines the overall size and energy of an orbital.
The energy of the electron on the outer shell the overall size of an atom the shape of the orbital the energy of an orbital the possible number of electrons on particular orbital the orientation of the orbital the overall size of an orbital. Chemists describe the shell and subshell in which an orbital belongs with a two-character code such as 2p or 4fThe first character indicates the shell n 2. Its possible values are n123 ldots ldots and so on.
What does the magnetic quantum number determine. Specifies the orientation of the orbital. He described the orbitals found in an Adam ER where the electron orbitals are within an atom.
In addition the greater the angular momentum quantum number the greater is the angular momentum of an electron at this orbital. Orbitals that have the same value of the principal quantum number form a shellOrbitals within a shell are divided into subshells that have the same value of the angular quantum number. The orbitals with higher values of n have greater energies than the orbitals with the lower values of n.
Answer 1 of 2. Check all that apply. In quantum mechanics the principal quantum number symbolized n is one of four quantum numbers assigned to each electron in an atom to describe that electrons state.
Orbitals with the same value of l form a subshell. Join our Discord to connect with other students 247 any time night or day. Apart from the principal quantum number the other quantum numbers for bound electrons are the azimuthal quantum number ℓ the.
The principal quantum number defines the general size and energy of the orbital. The first quantum number describes the electron shell or energy level of an atom. The pairing of.

The Principal Quantum Number N Youtube

How Many Different Values Of L Are Possible In The Third Principal Level In 2022 Different Values Principal Quantum

Quantum Numbers 3 7 Quantum Numbers Are Required To Describe The Distribution Of Electron Density In An Atom There Are Three Quantum Numbers Necessary Ppt Download

Question Video Using Quantum Numbers To Determine The Maximum Number Of Electrons In A Subshell Nagwa

Question Video Determining How Many Orbitals An Electron Could Be In Given The Principal And Subsidiary Quantum Numbers Nagwa
Solved What Does The Principal Quantum Number Determine Chegg Com

For Principle Quantum Number N 4 The Total Number Of Orbitals Having L 3 Youtube
What Does Principal Quantum Number Determine Psiberg
Quantum Numbers 3 7 Quantum Numbers Are Required To Describe The Distribution Of Electron Density In An Atom There Are Three Quantum Numbers Necessary Ppt Download
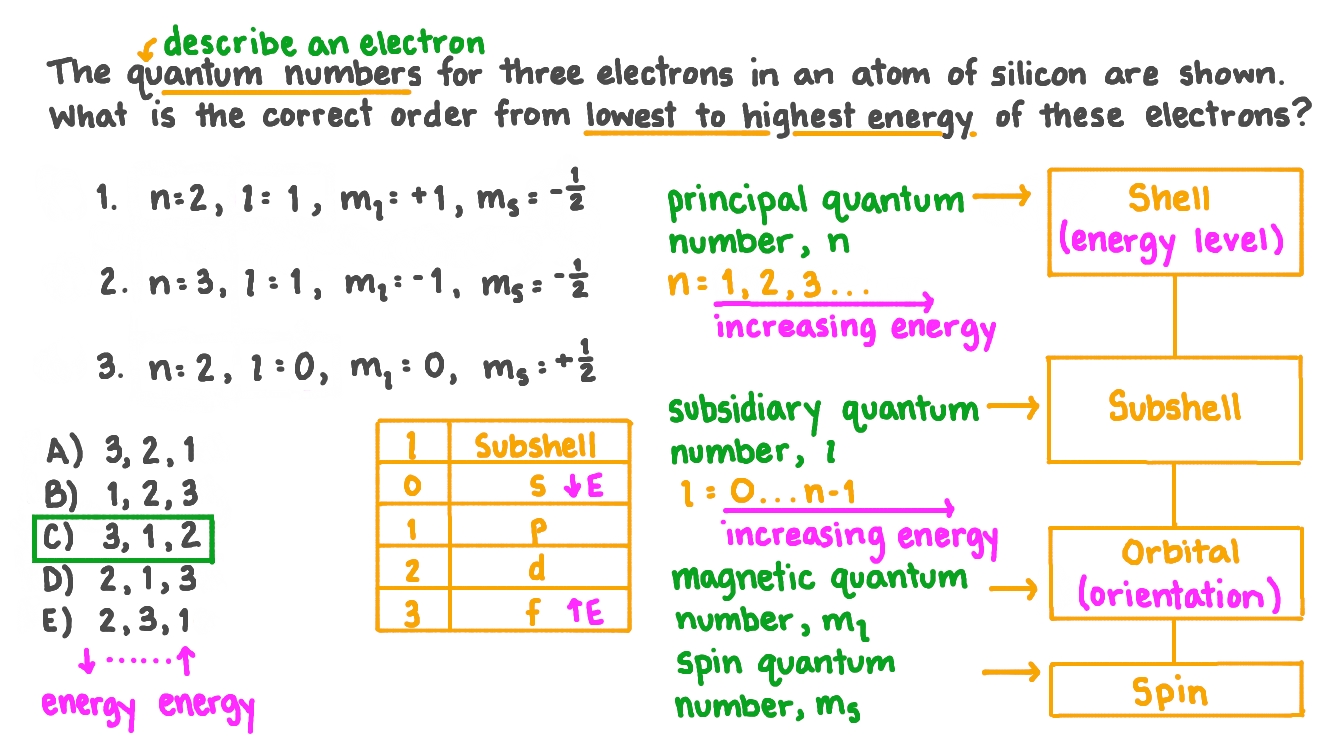
Question Video Ranking Three Electrons From Lowest To Highest Energy Given The Quantum Numbers Of Each Nagwa

Principal Quantum Number An Overview Sciencedirect Topics

Question Video Determining The Number Of Electrons Using Two Quantum Numbers Nagwa

Question Video Determining How Many Electrons In An Atom Can Have A Principal Quantum Number Of One Nagwa
Can You Explain The Significance Of 4 Quantum Numbers Quora

If The Value Of Principal Quantum Number Is 4 The Possible Values For Magnetic Quantum Youtube

Question Video Calculating The Number Of Atomic Orbitals From The Principal Quantum Number Nagwa

Principal Quantum Number Definition Determination Value

Question Video Determining The Quantum Numbers That Represent An Electron In An Atom Nagwa

Chemistry Quantum Numbers Chemistry Lessons Chemistry Classroom Chemistry

Comments
Post a Comment